Current Research Projects
One of the fascinating things about living systems is how readily they can respond to stimuli in a way that is both quick and reversible, allowing them to easily adjust to changing conditions. Rather than relying on the covalent bonds that hold most of our biological machinery together, these interactions rely largely on non-covalent bonding. My research looks at using non-covalent strategies to design sensors that mimic the fluid responsiveness of natural systems but yield fluorescent signals. We go about this using two main strategies, illustrated to the right. The first is to use triggered conformational changes in quadruplex nucleic acids, and the second is to harness the changes to the local environment that occur on encapsulation of a molecule in a synthetic capsule. These strategies are particularly interesting because they not only provide a new approach for developing useful sensors, but help us to understand some of the fundamental molecular interactions that drive both nucleic acid folding and the brilliance of fluorescent proteins.
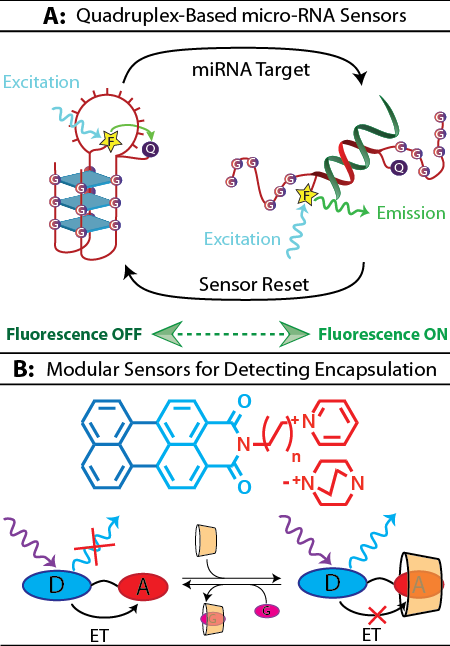
Quadruplex-based miRNA Sensors
The first approach (at the top of the figure to the right) looks at the folding of DNA as the basis for a sensors- in this case, we're interested in using the sensor to detect micro-RNA as a means of identifying cancer cells. Specifically, the sensor uses the ability of DNA to fold into multiple 3D structures- we start with a DNA quadruplex (on the left), and in the presence of our target micro-RNA, the sensor interacts to forma DNA duplex (on the right). This is associated with a fluorescent signal, as the fluorescence of the quadruplex is "off", and the structural transition to the duplex turns it "on". In addition to letting us design new sensors, this project also lets us better understand how DNA interconverts between quadruplex and duplex structures, a process that has been increasingly associated with gene regulation in promotor regions. As we gain a better understanding of quadruplex stability and how best to effectively trigger transitions, we can design increasingly effective and specific sensors. The use of the quadruplex is particularly beneficial, as it lets us design a "modular" sensor- one that we can easily update to respond to a new micro-RNA target- without the need for a complete redesign. Long-term, this project has the potential to give us flexible and stable fluorescent sensors that can detect cancerous cells by micro-RNA before a tumor has fully formed. With further development, the same platform could also be used for the detection of viruses through viral RNA.
Modular Sensors for Detecting Encapsulation
The second project (at the bottom of the figure to the right) looks at encapsulation as the basis for a set of sensors. Specifically, we're interested in the ability to detect the ability of a molecular capsule to bind any small molecule of interest, from medicine to contaminants. The idea centers on the changes that occur when a molecule (in this case, dyes that we synthesize in the lab) is encapsulated by the molecular capsule- a process we refer to as host-guest chemistry. Going from being surrounded by water (an aqueous environment) to being encapsulated changes the "environment" of the molecule, which can change how it behaves- and one of the changes in behavior is how brightly a molecule fluoresces. The dyes we synthesize are a series of rylene imide scaffolds (D, shown in blue) which act as electron donors, conjugated to different cationic amines (A, shown in red) which act as electron acceptors. When the dye isn't encapsulated (bottom left), the fluorescence of the rylene scaffold is shut off by an intramolecular oxidation/reduction reaction (charge transfer) between the two parts. When the molecule is encapsulated (bottom right), the change in environment shifts the reduction potential just enough that the charge transfer reaction is less favored than the fluorescent emission, and the fluorescence turns on. These dyes are useful as sensors because we can use them in "indicator displacement assays", where instead of looking directly at how a drug molecule binds a molecular capsule, we look at whether the drug (G, shown in purple) can "displace" our sensor from the capsule. This allows a single sensor to be used to test a wide range of drug-capsule binding interactions relatively easily. By developing and studying a range of these dyes and testing them in encapsulated and non-encapsulated states, we can better understand the effects of environment on the electrochemical and photophysical properties of organic molecules. A thorough understanding will let us develop better sensors, but we also hope will shed light on the function of fluorescent proteins, which function through an aromatic dye (made from amino acids) encapsulated in a protein capsule.